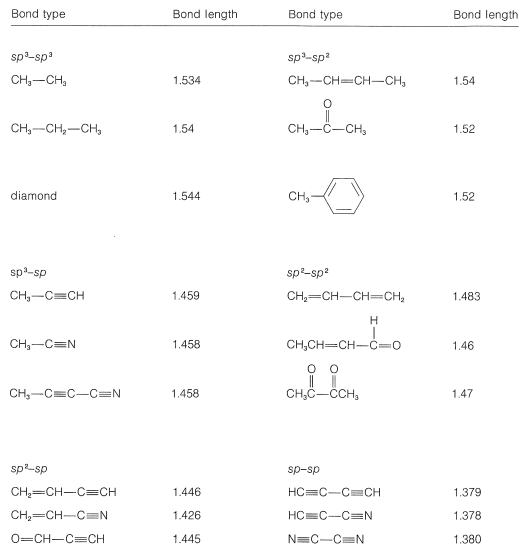
Optimized geometry and the relevant bond lengths of benzene molecule... | Download Scientific Diagram

Arenes. The story of benzene Write the electron configuration of Carbon. How many electrons are there in its outer shell? How many covalent bonds should. - ppt download
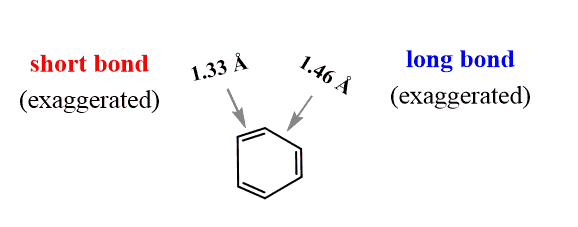
Give an account of expected and observed values of carbon-carbon bond lengths in benzene. - Sarthaks eConnect | Largest Online Education Community

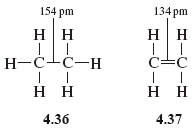
give reason C-C bond length in benzene ring is 139pm which is in between C-C single bond 154pm and C=C - Chemistry - Hydrocarbons - 3992697 | Meritnation.com
SOLVED: The carbon-to-carbon bond lengths in benzene, C6H6, are Select one: a. all equal to a typical C-C single bond b. all equal to a typical C=C double bond c. of different

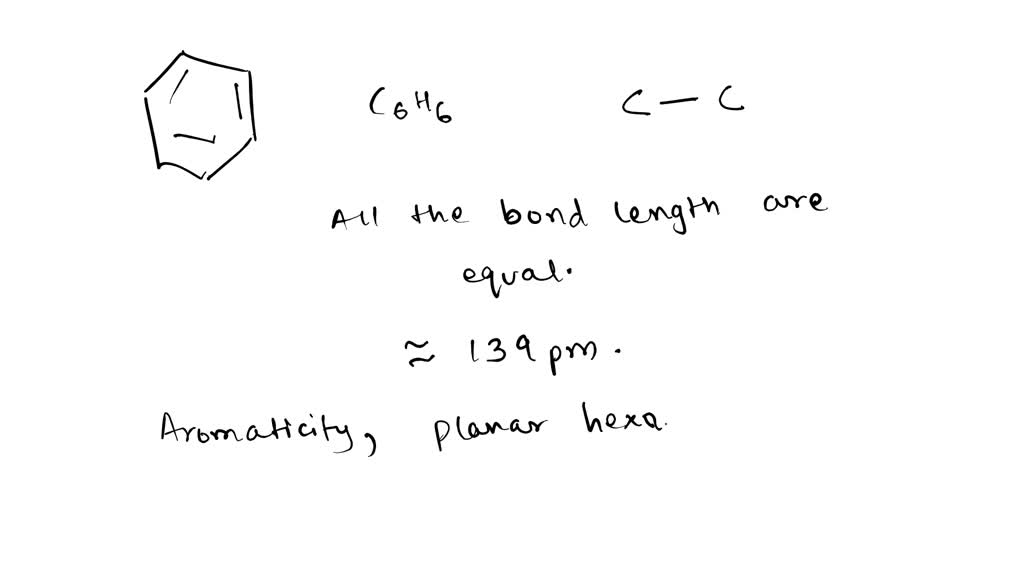
Benzene and the Concept of Aromaticity other representations: two equivalent resonance forms: - ppt download
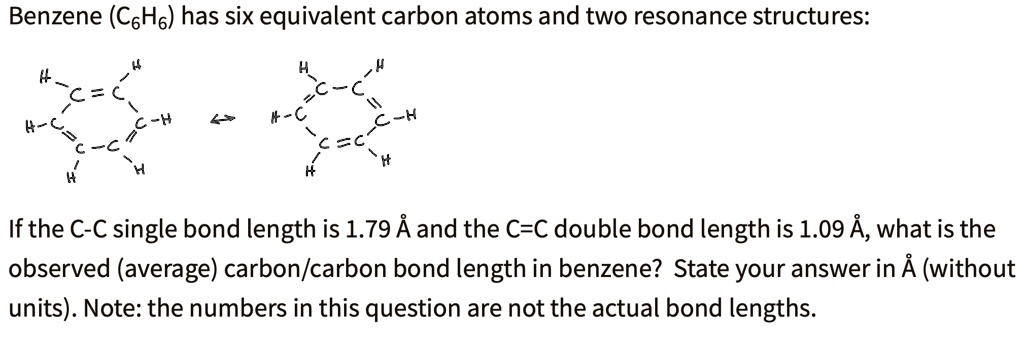


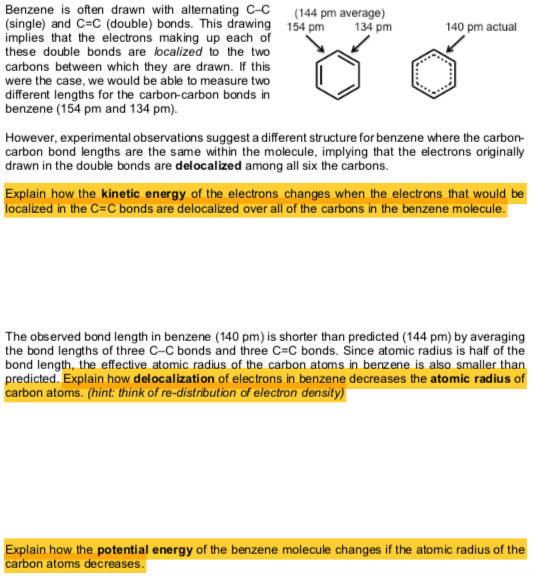

.bmp?revision=1&size=bestfit&width=356&height=210)