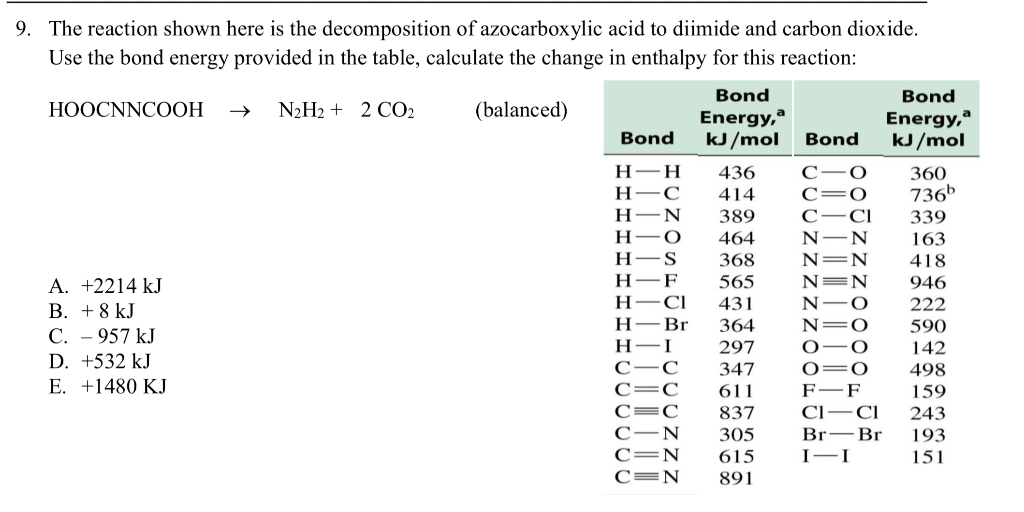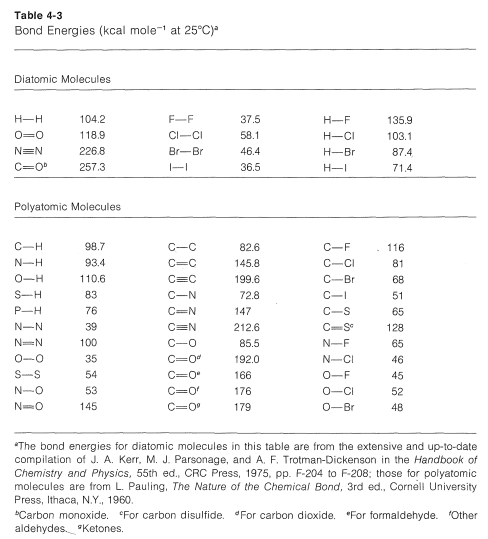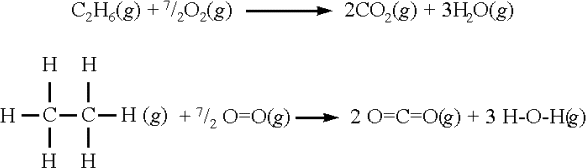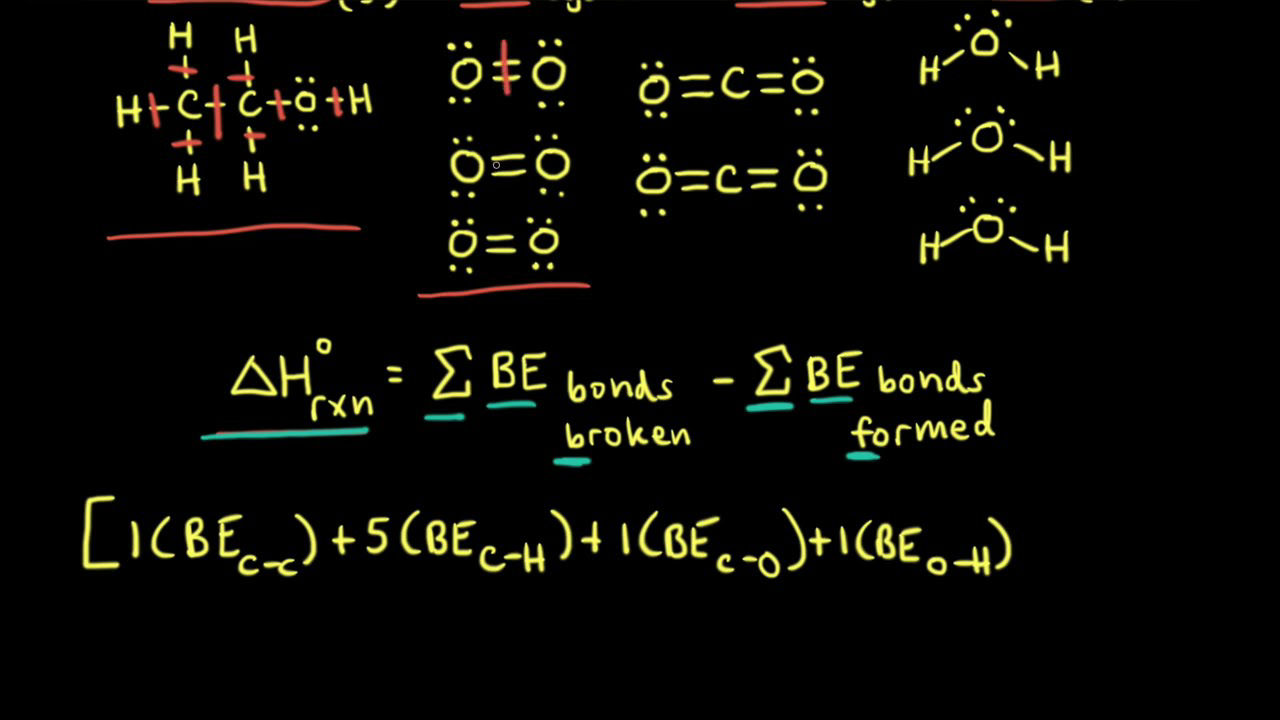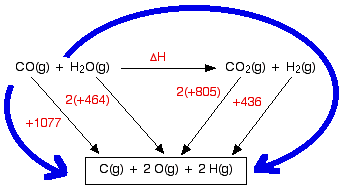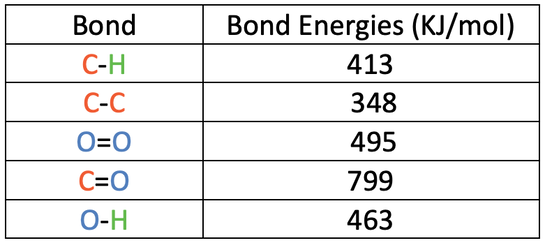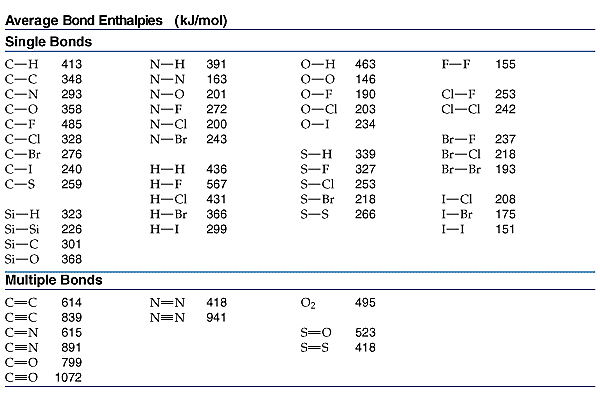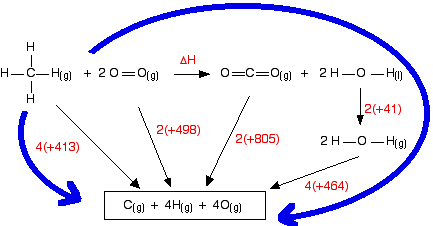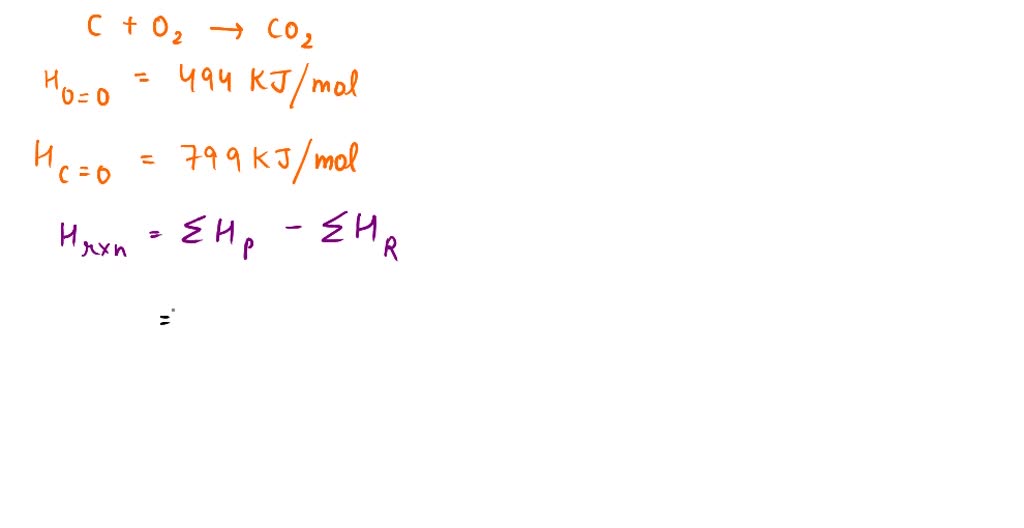
SOLVED: The bond enthalpy for an carbon-oxygen double bond in carbon dioxide is 799 kj mol-1. What is the enthalpy change for the following reaction? C(g) + 2O(g) >CO2(g) A -799kj B -

bond enthalpy/energy calculations for chemical reactions calculation of theoretical heat energy tranfers for exothermic or endothermic reactions gcse notes KS4 science igcse O level chemistry revision
A new approach to separate hydrogen from carbon dioxide using graphdiyne-like membrane | Scientific Reports
Active Thermochemical Tables: Sequential Bond Dissociation Enthalpies of Methane, Ethane, and Methanol and the Related Thermoche

physical chemistry - Why is the bond energy for a C=O bond higher in CO2? - Chemistry Stack Exchange

Energy Transfers Using a calorimeter Worked example: A fuel heated 40g of water which started at temperature of 20 degrees Celsius and finished at a temperature. - ppt download