Absolute and Relative Gas Concentration: Understanding Oxygen in Air Absolute amount of gas Relative amount of gas Effect of

The Henry's Law constant for oxygen dissolved in water is 4.34 xx 10 ^(4)atm ( mol /L) at 25^(@) C . If the partial pressure of oxygen in air is 0.2 atm
What is the number of moles of oxygen in one litre of air containing 21% of oxygen by volume under standard conditions? - Quora

Effect of NaOCl concentration (a) and flow rate of air oxygen (b) on... | Download Scientific Diagram

OneClass: What would be the saturation concentration (mole/L) of oxygen (O2) in a river in winter whe...
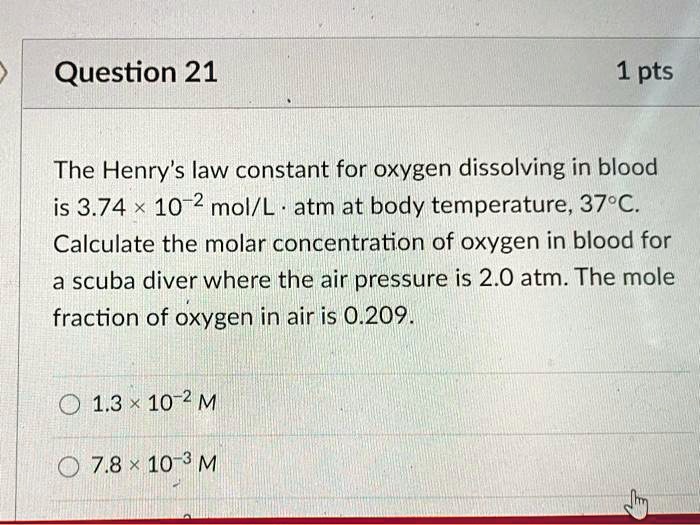
SOLVED: Question 21 1 pts The Henry's law constant for oxygen dissolving in blood is 3.74 10-2 mol/L atm at body temperature, 379C. Calculate the molar concentration of oxygen in blood for
What is the number of moles of oxygen in one litre of air containing 21% of oxygen by volume under standard conditions? - Quora

OneClass: Calculate the mass of oxygen gas (O_2) dissolved in a 5.00 L bucket of water exposed to a p...
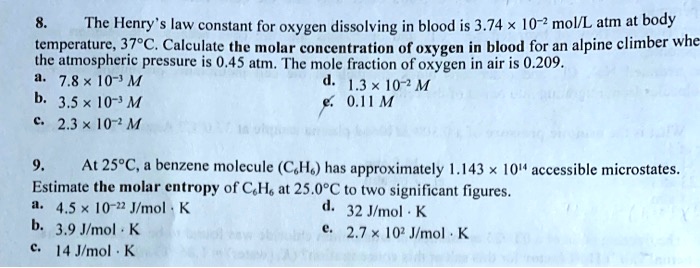
SOLVED: The Henry law constant for oxygen dissolving in blood is 3.74 x 10 ? molL atm at body temperature; 37PC. Calculate the molar concentration of oxygen in blood for an alpine

Henry's law constant for oxygen dissolved in water is 4.34 × 10^4 atm at 25^C . If the partial pressure of oxygen in air is 0.4atm. Calculate the concentration (in moles per