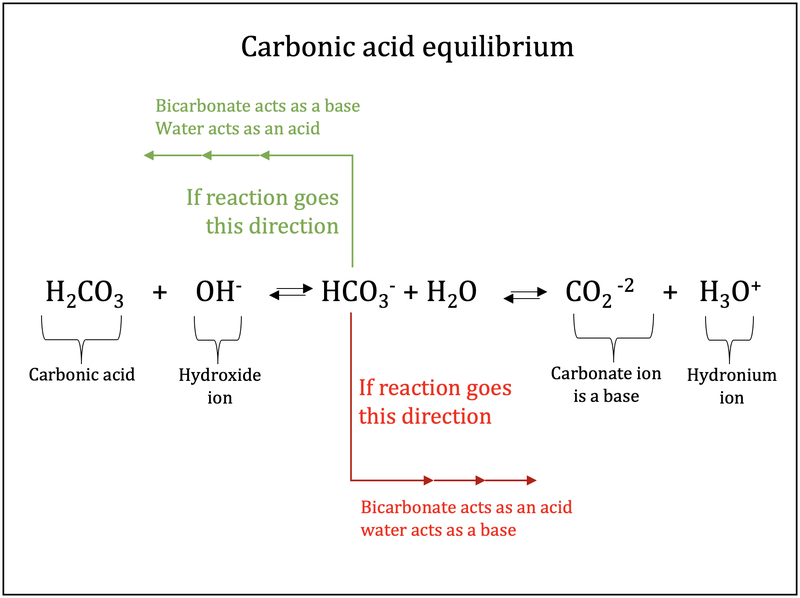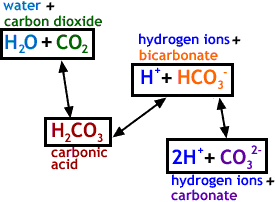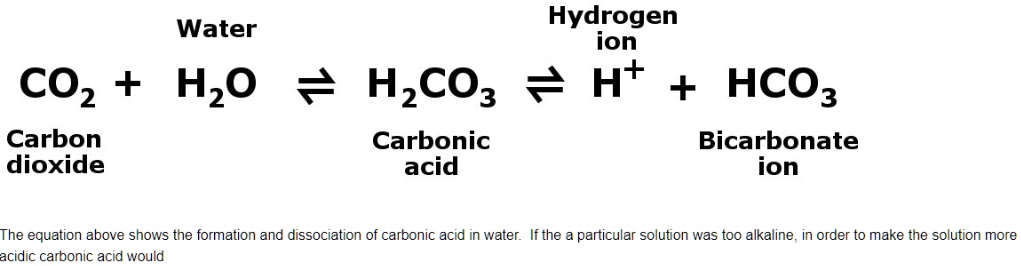
SOLVED: Water Hydrogen ion HzCO3 H+ HCO3 Carbonic Bicarbonate acid ion COz + Hzo Carbon dioxide The equation above shows the formation and dissociation of carbonic acid in water If the particular
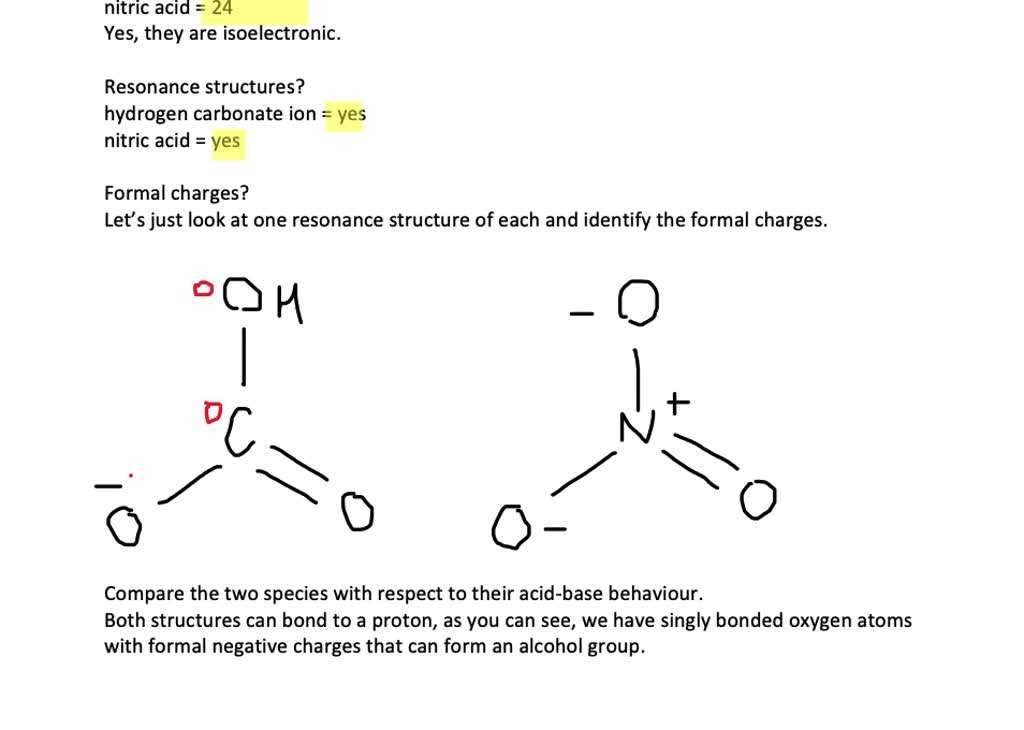
SOLVED:Compare the electron dot structures of the hydrogen carbonate ion and nitric acid. (a) Are these species isoelectronic? (b) How many resonance structures does each species have? (c) What are the formal

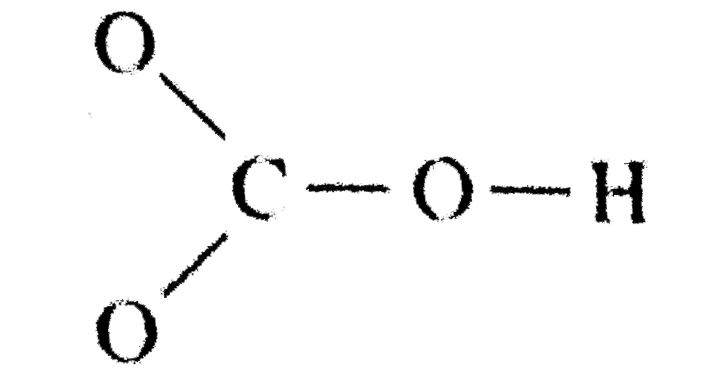
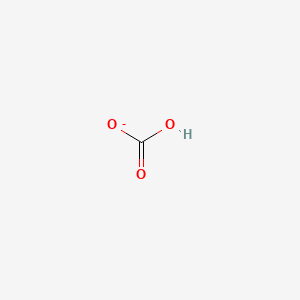
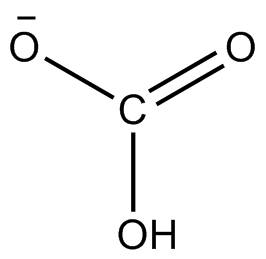
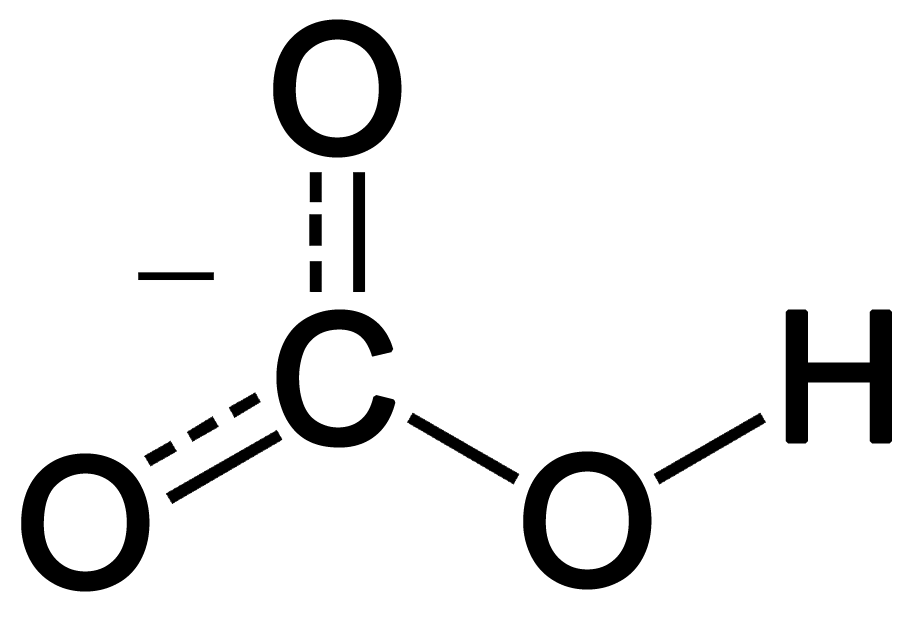
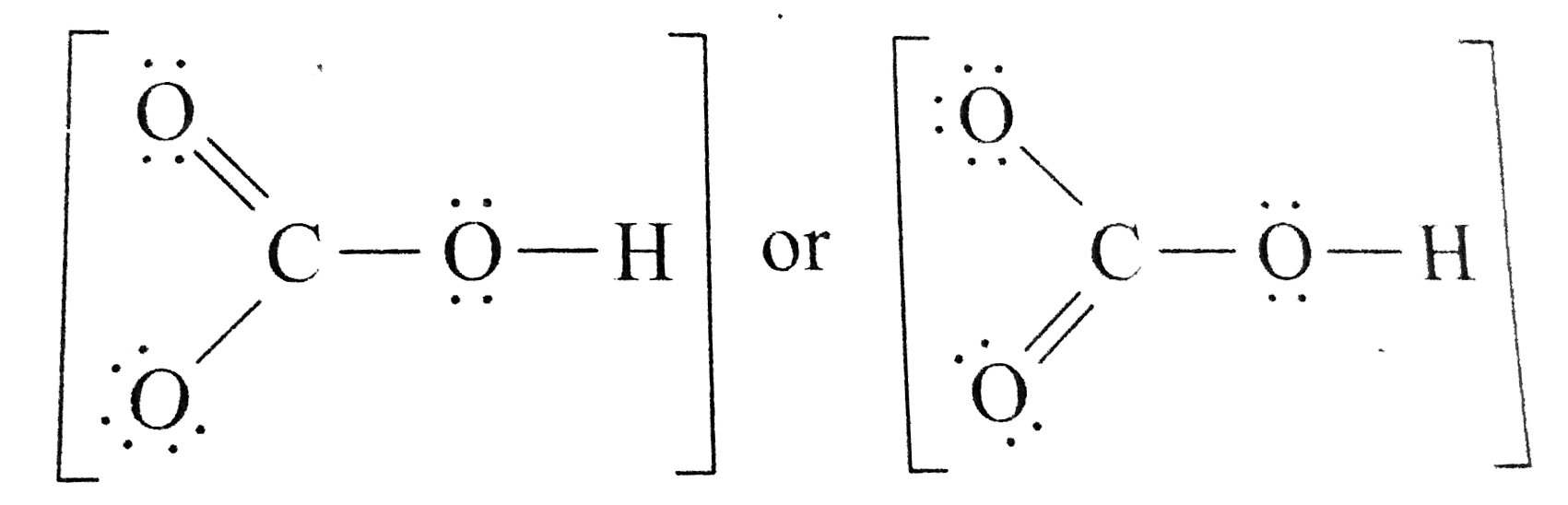
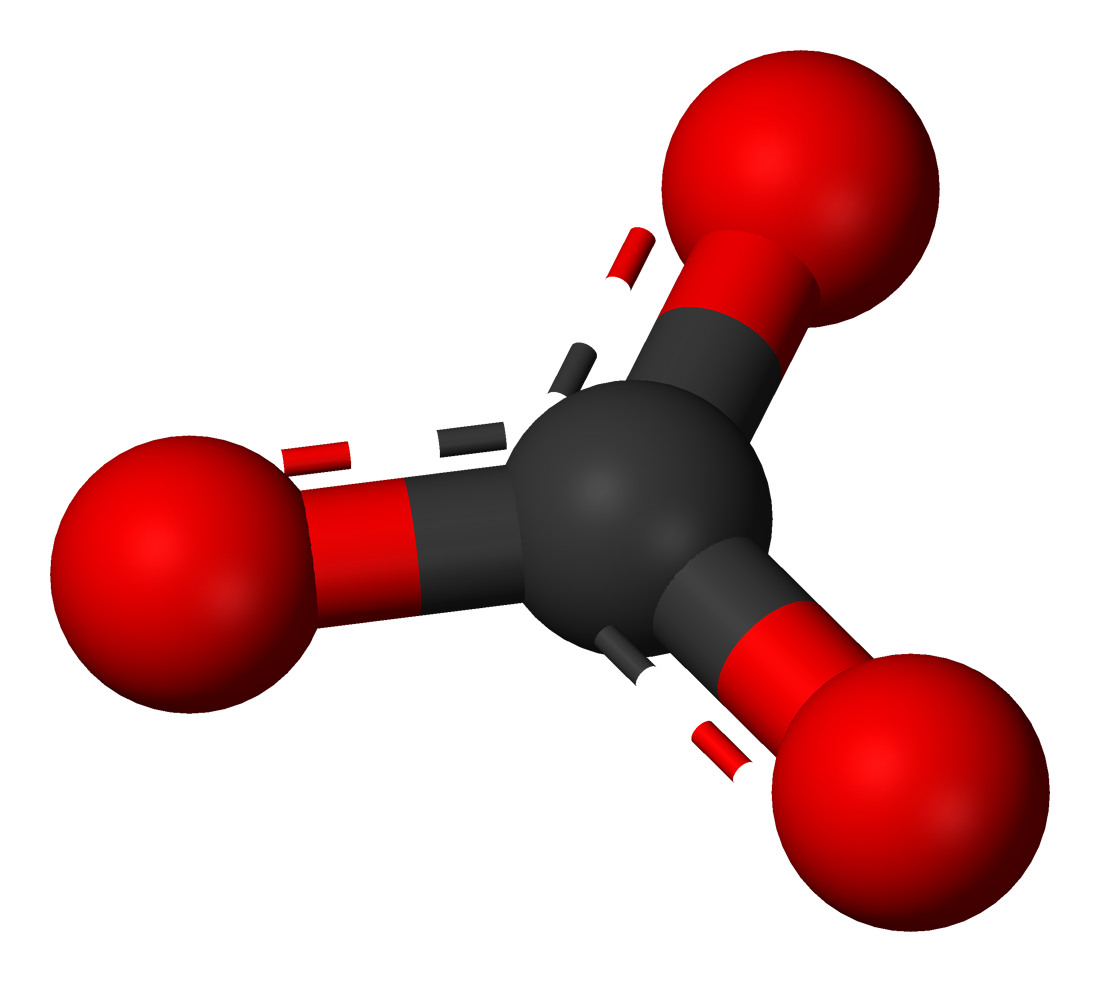
Toothpastes containing sodium hydrogen carbonate (sodium bicarbonate) and hydrogen peroxide are widely used. Write Lewis structures for the hydrogen carbonate ion and hydrogen peroxide molecule, with resonance forms where appropriate. | Homework.Study.com

acid base - In the bicarbonate ion, why can the hydrogen not bond to the carbon? - Chemistry Stack Exchange

Palladium-Catalyzed Deracemization of Allylic Carbonates in Water with Formation of Allylic Alcohols: Hydrogen Carbonate Ion as Nucleophile in the Palladium-Catalyzed Allylic Substitution and Kinetic Resolution | Journal of the American Chemical Society
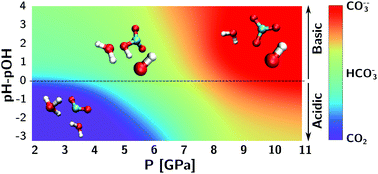
Carbon dioxide, bicarbonate and carbonate ions in aqueous solutions under deep Earth conditions - Physical Chemistry Chemical Physics (RSC Publishing)

a) The DFT structure of the isolated hydrogen carbonate. The symmetry... | Download Scientific Diagram