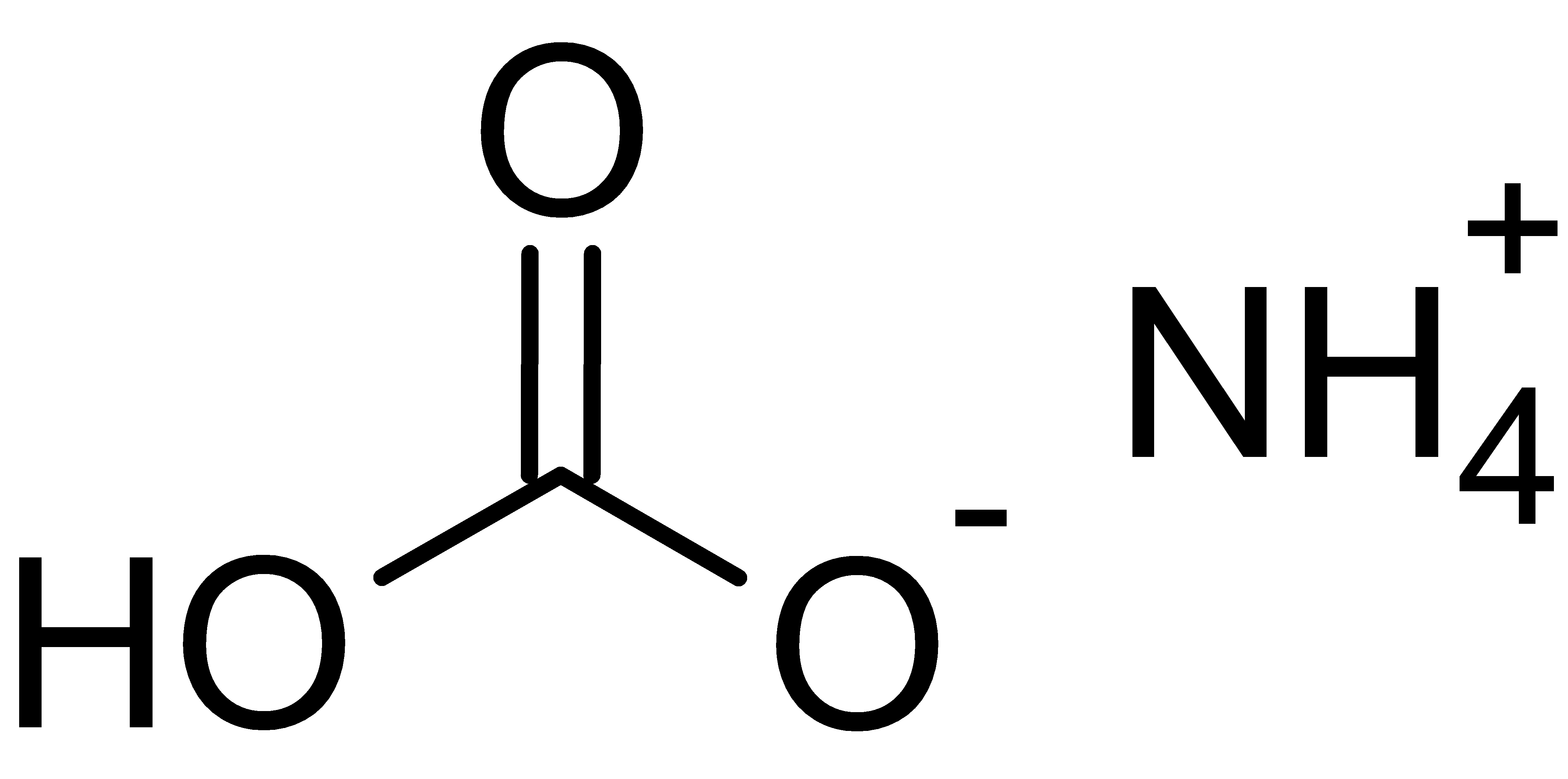
![If the relative molecular mass of ammonium nitrate is 80, the percentage of nitrogen and oxygen in ammonium nitrate is: [N = 14, H = 1, O = 16] If the relative molecular mass of ammonium nitrate is 80, the percentage of nitrogen and oxygen in ammonium nitrate is: [N = 14, H = 1, O = 16]](https://dwes9vv9u0550.cloudfront.net/images/3796715/307077b2-1bb0-4bde-a9d0-fe5f6659fec9.jpg)
If the relative molecular mass of ammonium nitrate is 80, the percentage of nitrogen and oxygen in ammonium nitrate is: [N = 14, H = 1, O = 16]
![Calculate the molecular mass of ammonium carbonate [(NH_4)_2CO_3] | 9 | THE LANGUAGE OF CHEMIST... - YouTube Calculate the molecular mass of ammonium carbonate [(NH_4)_2CO_3] | 9 | THE LANGUAGE OF CHEMIST... - YouTube](https://i.ytimg.com/vi/r17ijyWjA5c/maxresdefault.jpg)
Calculate the molecular mass of ammonium carbonate [(NH_4)_2CO_3] | 9 | THE LANGUAGE OF CHEMIST... - YouTube
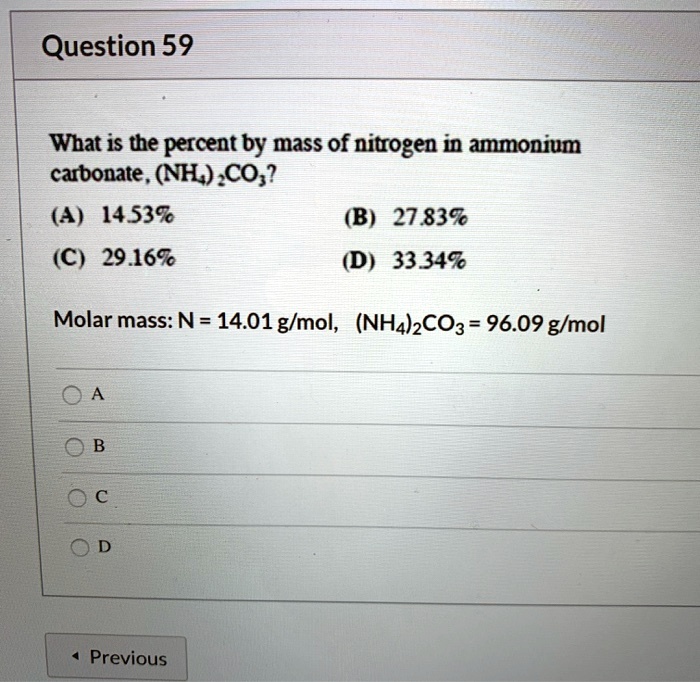
SOLVED: Question 59 What is the percent by mass of nitrogen in ammonium carbonate , (NH) CO;? (A) 1539 (B) 27,839 (C) 29.169 (D) 33.349 Molar mass: N= 14.01 g/mol, (NHA)2CO3 = 96.09 g/mol Previous
![If the relative molecular mass of ammonium nitrate is 80, the percentage of nitrogen and oxygen in ammonium nitrate is: [N = 14, H = 1, O = 16] If the relative molecular mass of ammonium nitrate is 80, the percentage of nitrogen and oxygen in ammonium nitrate is: [N = 14, H = 1, O = 16]](https://dwes9vv9u0550.cloudfront.net/images/3796715/8626e678-2ba5-4250-95ab-c650f06815b4.jpg)
If the relative molecular mass of ammonium nitrate is 80, the percentage of nitrogen and oxygen in ammonium nitrate is: [N = 14, H = 1, O = 16]

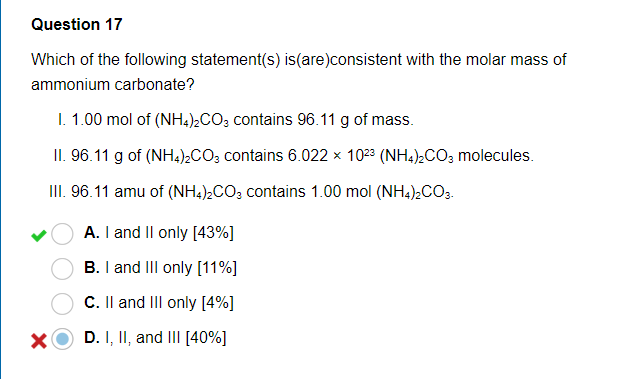
SOLVED: Ammonium carbonate, or (NH4 )2CO3 , a white solid that decomposes on warming, is a component of baking powder. a) How many formula units are in 41.6 g of (NH4 )2CO3 ?

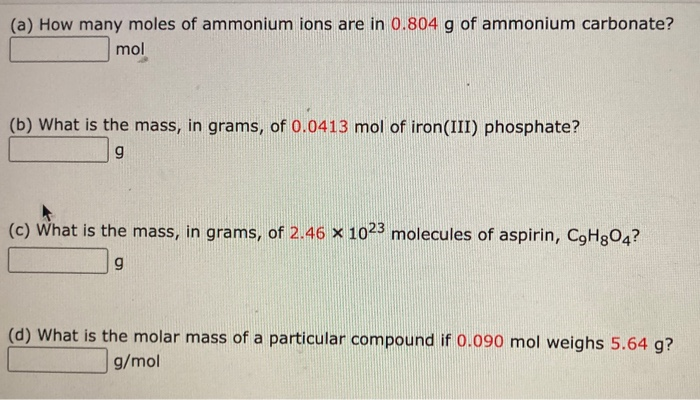
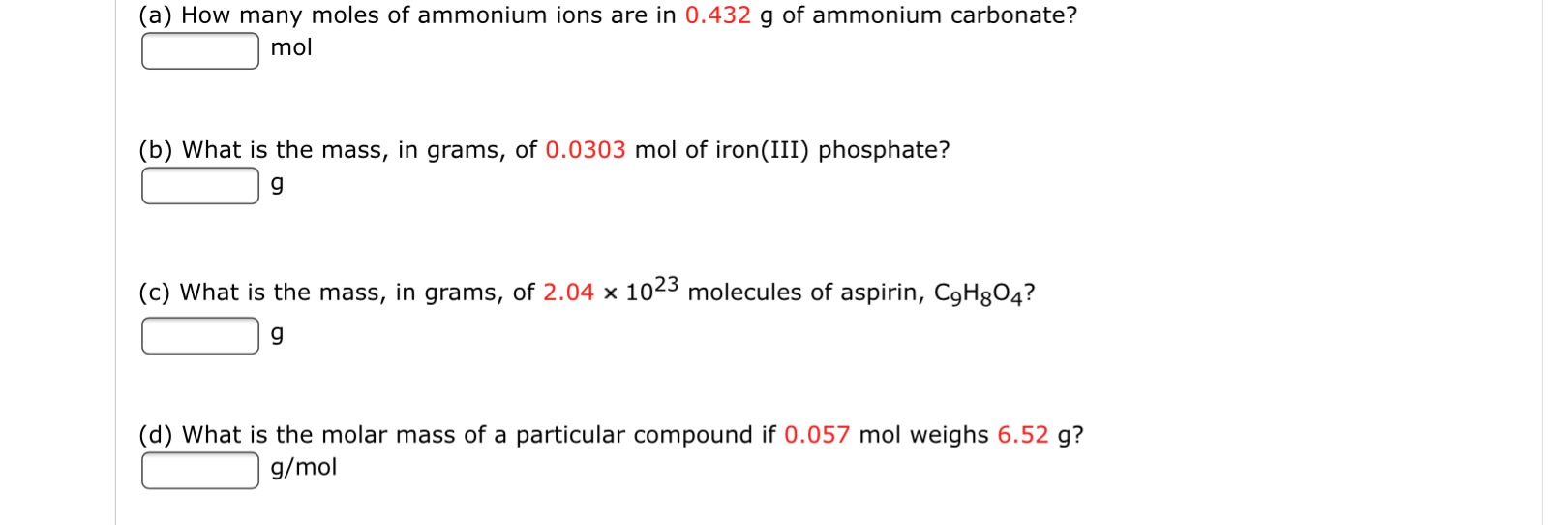
SOLVED: (a) How many moles of ammonium Ions are in 0.735 g of ammonium carbonate? 00382 mol (b) What is the mass, in grams, of 0.0670 mol of iron(III) phosphate? (c) What

The Mole Theory Molar Mass. Mass of a Mole o Molar Mass- the mass of a mole of any element or compound (in grams) o Also called: o Formula mass – sum. -

i) Calculate the molecular mass of ammonium carbonate ((NH4)2CO3).(ii) Find the percentage of nitrogen - Brainly.in

Calculate the molecular mass of ammonium carbonate (NHltsubgt4lt/subgt)ltsubgt2lt/subgt COltsubg... - YouTube
![If the relative molecular mass of ammonium nitrate is 80, the percentage of nitrogen and oxygen in ammonium nitrate is: [N = 14, H = 1, O = 16] If the relative molecular mass of ammonium nitrate is 80, the percentage of nitrogen and oxygen in ammonium nitrate is: [N = 14, H = 1, O = 16]](https://dwes9vv9u0550.cloudfront.net/images/9135167/05493bd1-a1eb-4ed3-85cf-f1917c8168b7.jpg)



![Calculate the molecular mass of ammonium carbonate [(NH4)2CO3] Calculate the molecular mass of ammonium carbonate [(NH4)2CO3]](https://d10lpgp6xz60nq.cloudfront.net/web-thumb/643924779_web.png)