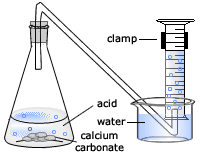
calcium carbonate reacts with dilute hydrochloric acid to produce carbon dioxide Stock Photo - Alamy

In an experiment, hydrochloric acid was added to calcium carbonate. These are the equations I came up with. Can somebody check them for me? Additionally, I'm thinking the net ionic equation is

The effect of changing the concentration of hydrochloric acid on the rate of reaction with calcium carbonate - IG Exams

Describe an experiment to study the speed of reaction between calcium carbonate and dilute hydrochloric acid, by measuring the loss in mass of reaction system over time. - Study notes, tips, worksheets,
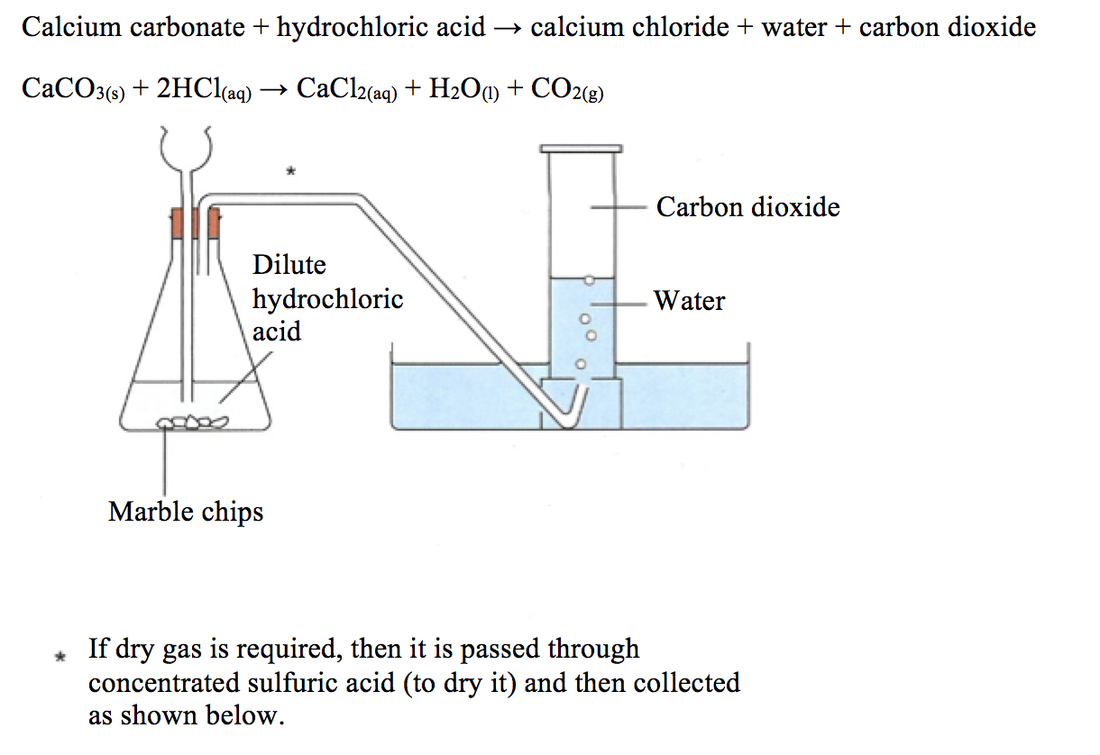
Q. Calcium carbonate reacts with aqueous HCL to give Calcium chloride and carbon dioxide ,according to the reaction CaCo3(s)+2HCl(aq) →CaCl2(aq)+CO2(g)+H2O(l) What mass of calcium carbonate is required to react completely with 25

a) Substitute formulae for names and balance the following equation: Calcium carbonate reacts - YouTube

Chemical reactions of two calcium salts (citrate and carbonate) with... | Download Scientific Diagram

CaCO3 + 2HCl → CaCl2 + H2O + CO2 The mass of calcium chloride formed when 2.5 g of calcium carbonate is dissolved in excess of hydrochloric acid is:

reaction mechanism - Calcium carbonate and hydrochloric acid - data analysis - Chemistry Stack Exchange