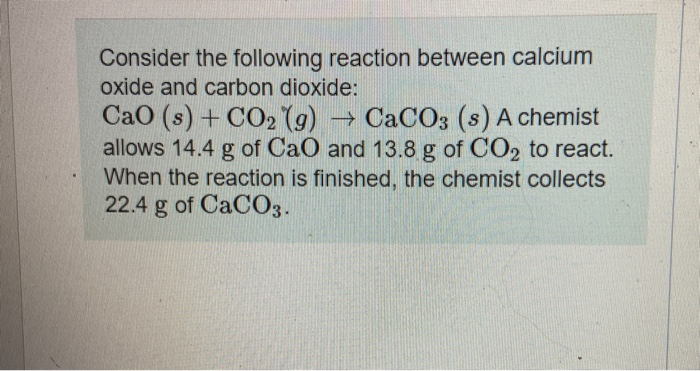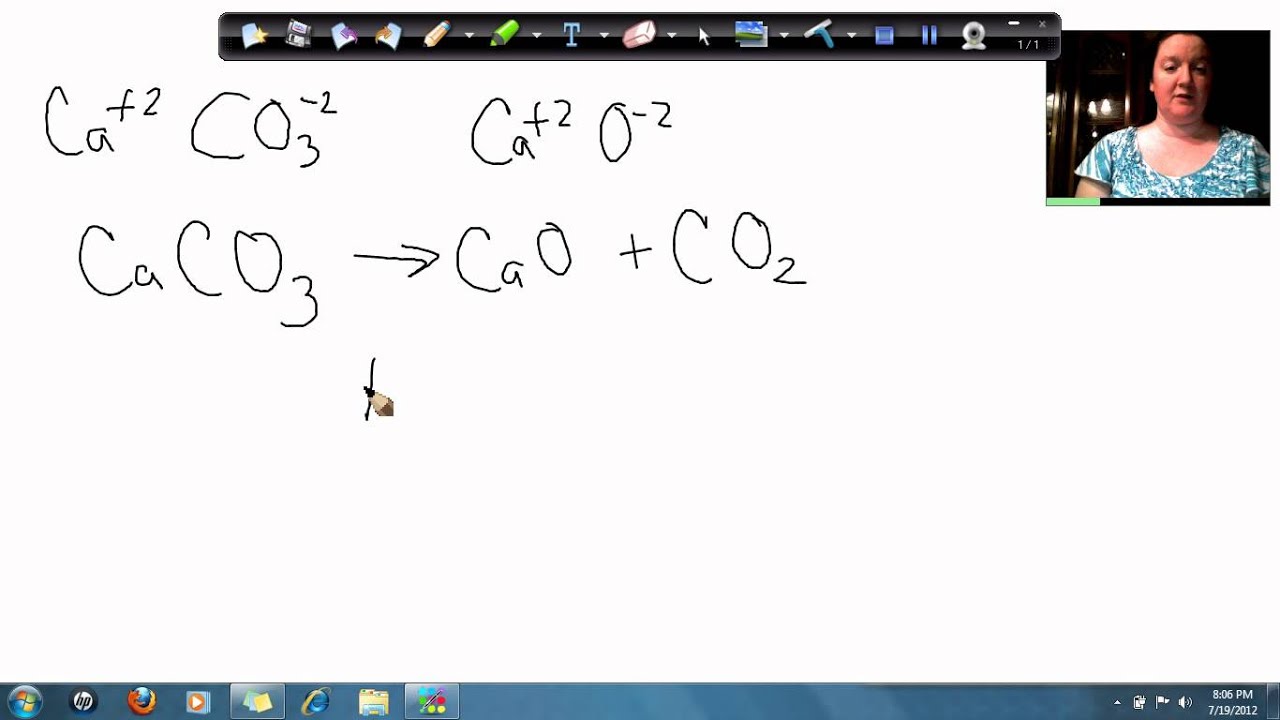Optimization of the structural characteristics of CaO and its effective stabilization yield high-capacity CO2 sorbents | Nature Communications

CaO+CO2=CaCO3 balance the chemical equation. Calcium oxide+carbon dioxide=calcium carbonate. - YouTube
Write the balanced chemical equations for the following reactions: i. Calcium hydroxide + Carbon dioxide → Calcium carbonate + Water - Sarthaks eConnect | Largest Online Education Community

Reaction steps involved in the formation of the CaCO3 sol from calcium... | Download Scientific Diagram

AQA GCSE Science & Additional Science Chemistry 1 Topic 2 Hodder Education Revision Lessons Limestone and building materials Click to continue. - ppt download

Calcium carbonate decomposes, on heating , to form calcium oxide and carbon dioxide. When 10 g of - YouTube

Question Video: Identifying the Chemical Equation- with State Symbols- That Corresponds to a Chemical Statement | Nagwa
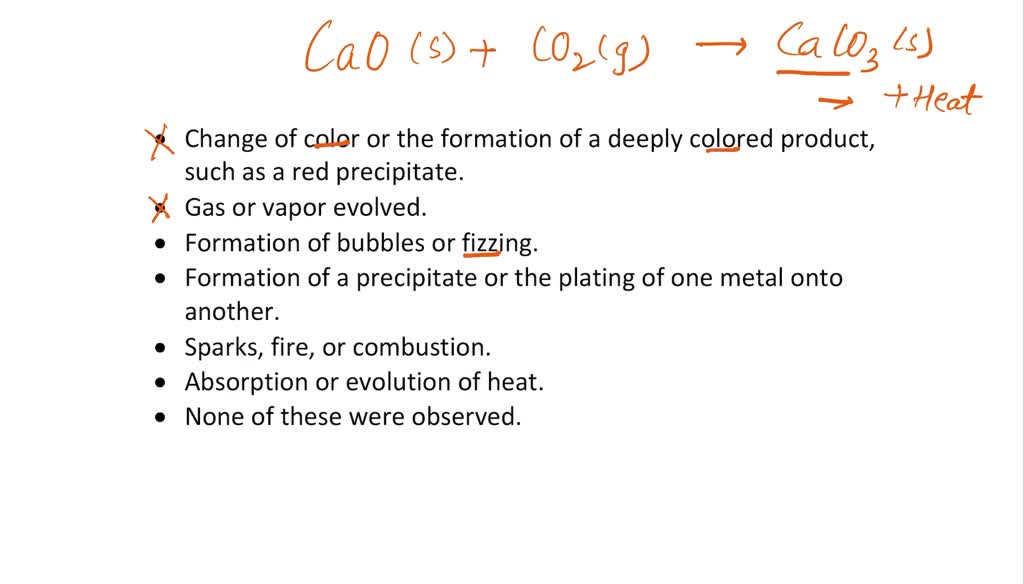
SOLVED: Reaction of calcium oxide and carbon dioxide: Watch the video for this experiment and list each of the changes you observed. Change of color or the formation of deeply colored product

Calcium carbonate decomposes on heating to give calcium oxide and carbon dioxide. How much volume of CO2 will be obtained by thermal decomposition of 50g CaCO3 ?