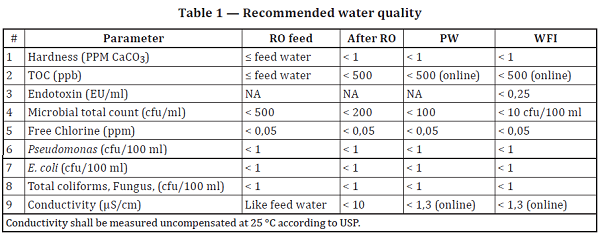
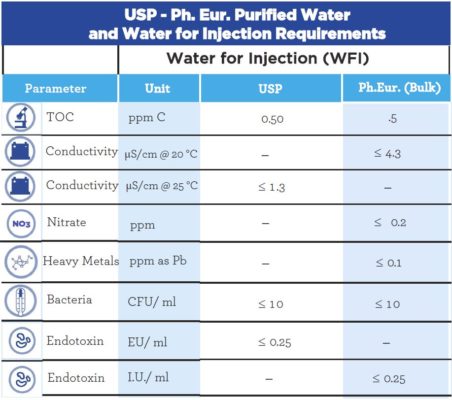
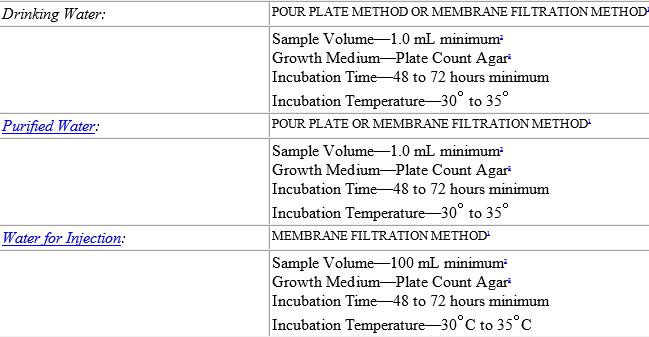
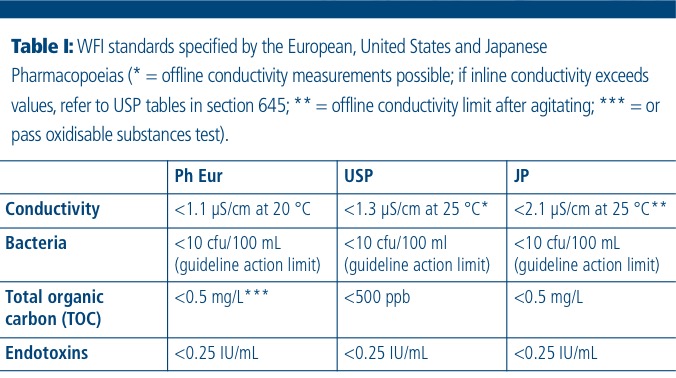
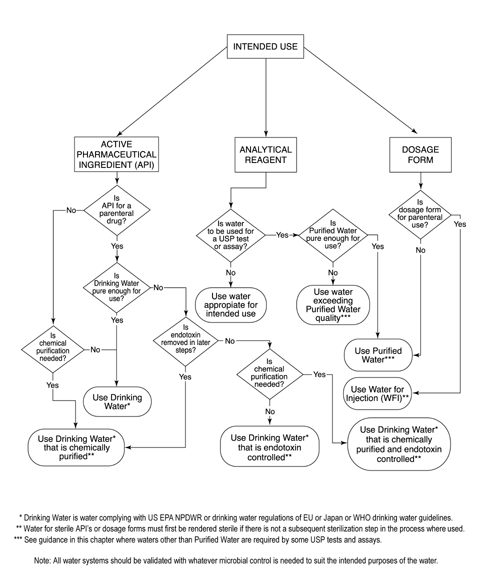
Testing of Purified Water, Raw Materials, In-Process Samples and Finished Non-Sterile Products | American Pharmaceutical Review - The Review of American Pharmaceutical Business & Technology
In the space of reconstitution and non-sterile compounding in the retail pharmacy world, water for use during reconstitution has